Note: This article is the fourth of seven articles comparing methods of protective metal finishing processes.
Part 1: CARC Environmentally Safe?
Part 2: Anodizing
Part 3: Galvanization
Part 4: Electroplating
Part 5: Autodeposition/A-Coating
Part 6: Powder Coating
Part 7: E-Coat vs A-Coat
Alchemy. What images popped into your head after reading that word? Did you see smoking cauldrons with people hovering over them with dour looks?
I don’t know about you, but I always thought alchemists weren’t straight in the head. The thought of changing one metal into gold seemed foolish.
Shows what I know. Alchemy became the basis for the evolution of modern science. Two of the most famous scientists in history were alchemists. Robert Boyle and Sir Isaac Newton were part of a process developing into things such as:
- Creating new alloys
- Manufacturing acids and pigments
- Inventing apparatus for distillation
- The process used in making perfumes and whiskeys
- Conceiving of atoms centuries before modern atomic theory
- Providing a template for the scientific method by running controlled experiments again and again
Electroplating is a child of alchemy. What is electroplating?
I’m happy you asked!
What is Electroplating?
Electroplating uses electric current reducing dissolved metal cations, developing a thin and coherent metal layer on the electrodes. This process forms silver chloride electrodes and beating the metal against each other, mainly preventing corrosion.
It also builds up thicknesses and produces electroplating objects. This process adds metals without these properties to surfaces with features such as hardness or strength.
Adding Current
 Electroplacement is using an electric current to coat or clad a metal layer on a surface or material. It changes the features of that material.
Electroplacement is using an electric current to coat or clad a metal layer on a surface or material. It changes the features of that material.
Depending on the desired chemical, physical, or mechanical properties, they use several metals for electroplating.
Electroplating is like electrolysis—they both use electricity, splitting the solution. But electrolysis produces current while electroplating requires current. However, both processes belong to the same branch of chemistry called electrochemistry.
The Electroplating Process
As mentioned before, electrochemistry connects a very thin layer of a selected metal with the surface of another metal at the molecular level. Electroplating is possible with a variety of metals such as copper, silver, gold, copper oxide, and lead.
 The electroplating process is comparatively fast and straightforward. It doesn’t require enormous capital investments.
The electroplating process is comparatively fast and straightforward. It doesn’t require enormous capital investments.
The first step in the process is choosing the right electrodes and electrolyte solution. The key is understanding the chemical reaction when passing current between them. It’s essential to factor in that the metal atoms that work towards plating the object comes directly from the electrolyte. This means if you want to plate copper, then the solution contains copper sulfate. If silver, then silver salt; for gold plating, a gold-based electrolyte.
After choosing the electrodes and solution, cleaning becomes necessary. If the electrodes aren’t clean, then the metal atoms deposited on it won’t stick. The atoms rub off while removing the solution. Cleaning means dipping the electrode into a powerful acid or some alkaline solution like potassium hydroxide.
Main Electroplating
Electroplating is the second phase requiring two electrodes comprising different materials. For the process to work, one electrode must be the metal you’re plating, the other a salt solution. For instance, if you are plating copper on brass, there are copper and brass electrodes, both dipped in a copper sulfate solution.
The challenging part is that metals like silver and gold don’t easily dissolve; they require unpleasant and highly dangerous cyanide-based salts. The electroplating metal is usually a very cheap metal or even a nonmetal coated with some conductive coating like graphite. Regardless, the goal is to conduct electricity, because, without it, the electroplating process is unsuccessful.
Charging the metals is the job of a power source like a battery or power supply. Electricity flowing through the manufacturing circuit splits and galvanizes the electrolytes. It deposits the metals in thin layers at the top and bottom of an electrode.
Electrodes
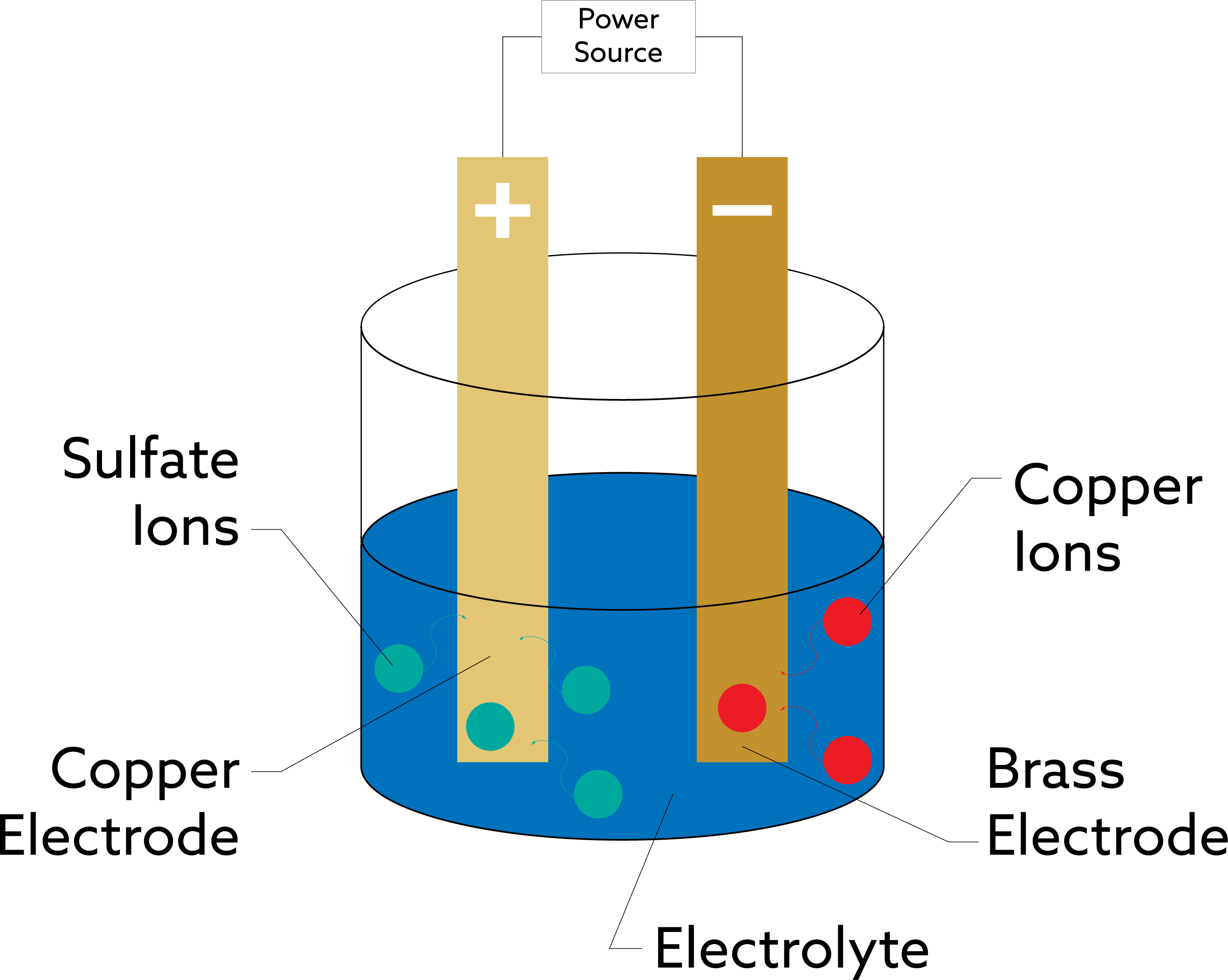 The electrodes are then dipped and connected into the solution. The electrodes use a circuit so that the copper electrode becomes the positive terminal or anode. In contrast, the brass becomes the circuit’s negative terminal or cathode. Turning power on splits the copper sulfate solution into ions. The copper ions with a positive charge will move towards the brass electrode and deposit there, resulting in a thin layer. The sulfate moves toward the copper electrode, and over time coats the copper electrode with a green sheet, becoming copper sulfate.
The electrodes are then dipped and connected into the solution. The electrodes use a circuit so that the copper electrode becomes the positive terminal or anode. In contrast, the brass becomes the circuit’s negative terminal or cathode. Turning power on splits the copper sulfate solution into ions. The copper ions with a positive charge will move towards the brass electrode and deposit there, resulting in a thin layer. The sulfate moves toward the copper electrode, and over time coats the copper electrode with a green sheet, becoming copper sulfate.
The process takes a bit of time, and the entire build-up, depending on the scale of the system, takes a few hours. The speed at which electroplating occurs also depends on the intensity of the current. So, increasing either the current or the concentration of the electrolyte has a direct effect on how soon the process is complete. Also, as long as the current keeps flowing, as the circuit is complete, the electroplating process will progress.
In Sum
Alchemy started independently in ancient Egypt, India, and China, more like a religious act. It developed and advanced the theory of chemical processes. Our modern world wouldn’t be so modern without those ancient alchemists.
I imagine if Newton magically appeared today, he’d be stunned by modern-day alchemy. Well, after seeing planes, trains, and automobiles, of course, he’d be amazed.